Projects
Cancer prevention
Scientific Epidemiological Study Center

From left to right: (rear) Dipl. Stat. Antje Müller, Dipl. Stat. Isabelle Groß, Susanne Posse, Dipl. Stat. Nadine Bonberg, Adam Lanzer, (front): Gabriele Engelhardt, Helga Nauen, Prof. Dr. Thomas Behrens, Sigrid Semke, Dipl. Soz. Jan Hovanec.
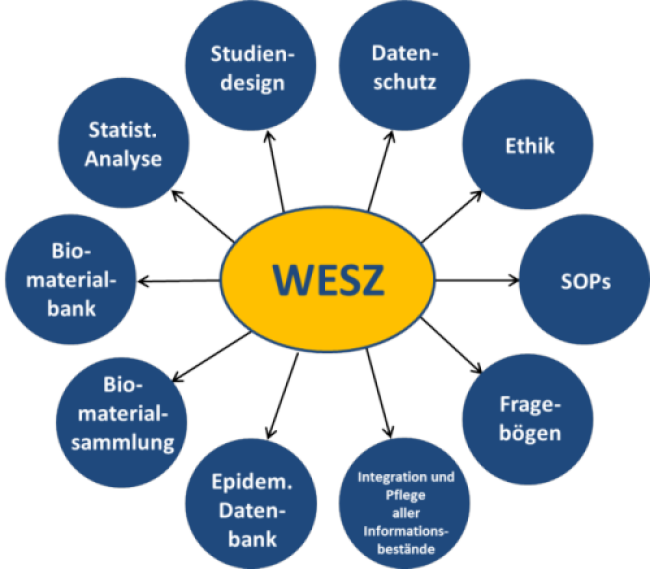
The duties of the scientific epidemiological study center comprise all fields of study coordination and implementation of rules for good epidemiological practice.
These involve amongst others:
- conceptual design of ethics proposal
- design of concepts for data privacy protection, including a trustee model (in cooperation with the Medical Council Westfalen-Lippe)
- design of clinical study protocols, operation manuals and standard operating procedures (SOPs)
- operation of a biomaterial bank (including organization and coordination of sample collection)
- interviews
- maintenance and documentation of project-based pathological, clinical and epidemiological data sets
- statistical and epidemiological evaluation
The scientific epidemiological study center provides proper conditions for the studies and thus the validity and sustainability of research results.
To improve diagnostic, therapeutic and predictive value of biomarkers, candidate biomarkers and marker-panels in PURE are validated in a second set of test persons.
By providing proper surveys of test persons (regarding data-protection law) and quality-assured acquisition of bio samples (tissue, blood, urea) in accordance with legal, ethical and epidemiological standards as well as installation and maintenance of the biobank the scientific epidemiological study center lays a solid foundation for all research groups in PURE. Beyond this we focus on accelerating the identification and validation of biomarkers by statistical and epidemiological means and on ensuring the clinical-epidemiological follow-up of patients.
Biobank
The scientific epidemiological study center (WESZ) organizes and coordinates the systematic collection, storage and distribution of frozen and paraffin embedded tissue samples as well as blood and urine within the different locations of PURE.
We are using the software STARLIMS to manage present and future organizational, logistical and technical processes. STARLIMS enables to transparently record the distribution, the usage and the whereabouts of samples in stock. The corresponding database meets the ethical and data privacy protection criteria of the Ruhr-University Bochum and of the State of North Rhine Westphalia.
The WESZ is currently working on a data warehouse to integrate pathological, clinical and epidemiological information in the sample specific database. This data warehouse will meet the high data security requirements of the TMF – the technology and methods platform for networked medical research in Germany. The listing in the German Biobank Register is another aspired milestone for the biobank. It is designed as a permanent institution of the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance (IPA).
Statistics
Statisticians of the WESZ are successfully involved in epidemiological studies for many years now. They evaluate information form questionnaires and analytical results together with scientists from the platform technologies. They also evaluate data for prognosis and epidemiological follow-up from clinics, residents´ registration offices and the ministry of health (medical condition, cause of death).
Study Nurses
Study Nurses of the WESZ are qualified and certified student assistants from clinical and epidemiological research. They are deployed in the organization and processing of the bio samples at cooperating clinics as well as the transport of samples to the biobank, the documentation of study relevant data and the data collection via questionnaires from the participating test subjects.
Further duties include the design of the questionnaires, the data collection (including plausibility checks) and quality management. Tasks associated with the conduction of the study, like the management and archiving of bio samples, the digitization of tissue sections and many more complete the duties of the study nurses.
Duties of the scientific epidemiological study center (WESZ)
-
Institute for Prevention and Occupational Medicine,
German Social Accident Insurance
Institute of the Ruhr University Bochum (IPA)
Prof. Dr. med. Thomas Behrens
Bürkle-de-la-Camp Platz 1
44789 Bochum
Tel. +49 (0)234 302-4794
Fax +49 (0)234 302-4609

